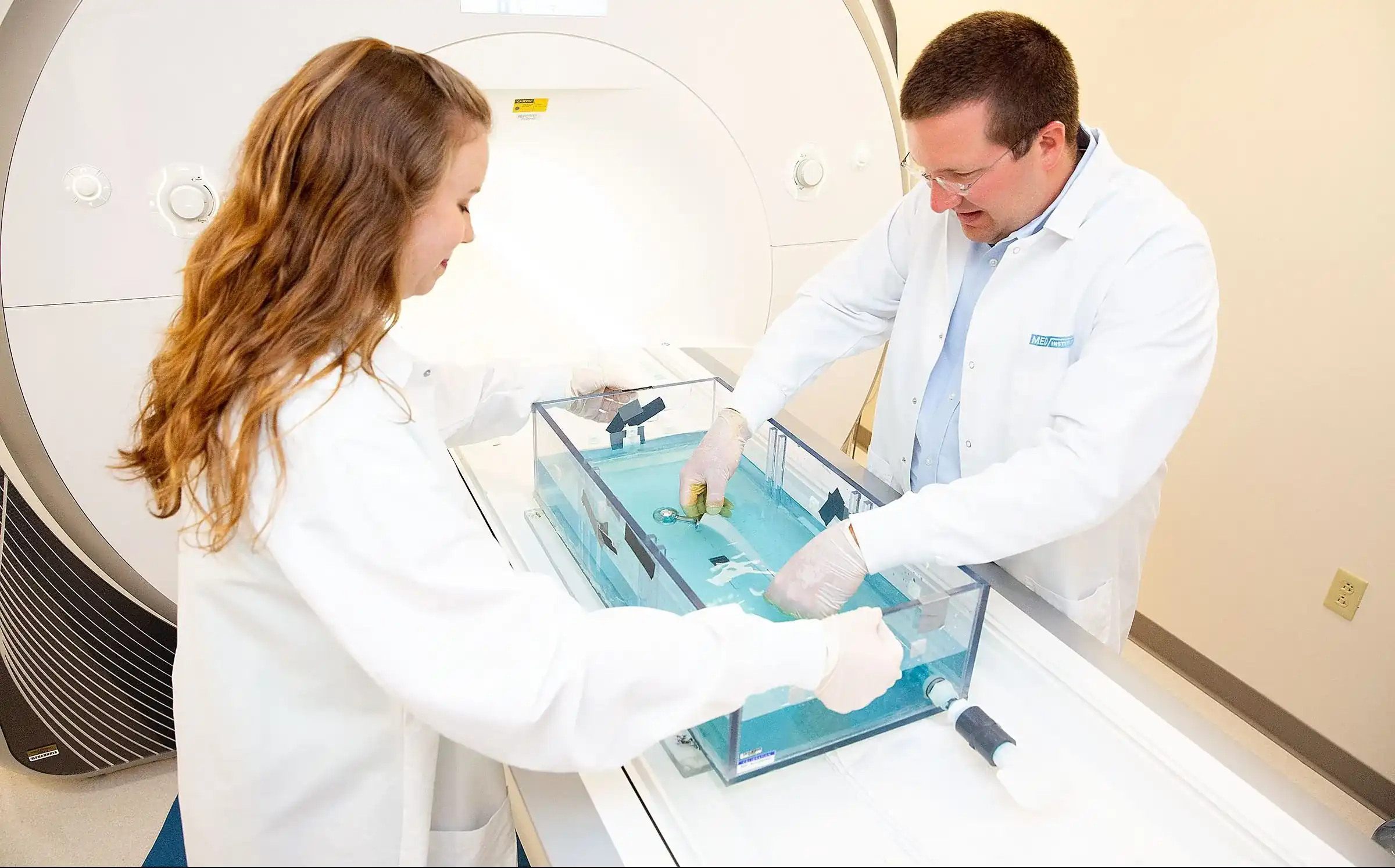
When you need a team with broad experience and knowledge who really wants to get your medical product idea to the market where it can help patients, we are ready to collaborate. Contact us to learn more and to discuss what we can do to help guide your project through the medical device product lifecycle.
Latest News
The latest news and thought leadership from MED Institute.
-

ISO 10993-1 Revision Highlights Part 4: Reinforcement of Material and Chemical Characterization
February 17, 2026Read previous posts in this series Part 1, Part 2, and part 3. The importance of material...
-

ISO 10993-1 Revision Highlights Part 3: Biological Effects
January 30, 2026Read part 1 and part 2. The sixth edition of ISO 10993-1(2025) introduces significant updates that align biological risk evaluation...
-

ISO 10993-1:2025 Revision Highlights Part 2: Exposure Duration = Exposure + Duration
December 3, 2025Calculation of Exposure Duration for Categorization of Medical Devices While exposure duration is not a new concept...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).