Medical device concept development
Developing medical devices
Concept development starts with a good idea, ideally directed toward an unmet clinical need. In making the leap from an idea to initial concept, we can begin to use engineering know how to develop the optimal medical device. Decisions and progress at this point can have a lasting impact on the future success of the medical device.
Our team has the training and experience to move a medical product from a raw idea to an initial functional concept, while balancing such important things as manufacturability and suitability for the clinical setting. We work closely with clinicians and inventors during this concept phase, observing and listening to understand the problem and to assess the proposed solution. We continue to refine and improve our approach to device development and understand this is not a one size fits all process. We have found value in refining our concept generation process, which has paid off in being able to move more quickly and efficiently through early phases of development. This flexibility has helped us develop user needs, refine business strategies to reach the development target, and leverage infrastructure and processes to move a technology forward. 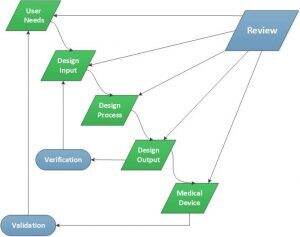
Supplementing client resources with MED resources is a natural fit for our company culture. We use a collaborative communication approach and have many options to fit your needs. We can communicate via phone/video calls, face to face meetings, or through our website if needed. The speed at which you can iterate through this early phase is important as it is time you cannot get back. Working with a wide range of clients, including large medical device manufacturers and university-based start-ups, has helped to define our development process. At times, gathering more requirements is necessary. For example, one client might need physical prototypes while another will be able to work with a computer-generated representation of the prototype. We work very closely with our regulatory staff to supply the appropriate regulatory strategy. We have an ISO 13485 quality system that can “slim down” during these early R&D phases. Budgets for start-up and early phase companies are often small, and we understand and can accommodate that situation. We enjoy the challenges of moving a technology through this phase of R&D.
Key Activities
Concept development, User Needs Identification, R & D, Innovation
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
