Medical Device Risk Analysis, Management, & Design Control
Engineering Services
Creating appropriate and regulated documentation of your device during development can be an overwhelming experience. There are several commercial software systems available to help manage your design history file (DHF) or alternatively, you can create a system designed for your needs. Regardless, developing a relationship with a partner that has managed creation of design controls through best practices is prudent. A good starting point is to go to the regulations, a small excerpt of critical sections of 21 CFR Title 820.30 are as follows:
“(a) General.
(1) Each manufacturer of any class III or class II device, and the class I devices listed in paragraph (a)(2) of this section, shall establish and maintain procedures to control the design of the device in order to ensure that specified design requirements are met.
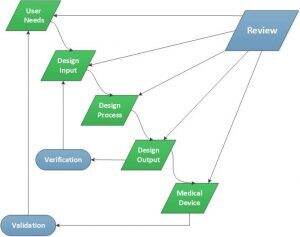
(b) Design and development planning. Each manufacturer shall establish and maintain plans that describe or reference the design and development activities and define responsibility for implementation. The plans shall identify and describe the interfaces with different groups or activities that provide, or result in, input to the design and development process. The plans shall be reviewed, updated, and approved as design and development evolves.
(c) Design input. Each manufacturer shall establish and maintain procedures to ensure that the design requirements relating to a device are appropriate and address the intended use of the device, including the needs of the user and patient. The procedures shall include a mechanism for addressing incomplete, ambiguous, or conflicting requirements. The design input requirements shall be documented and shall be reviewed and approved by a designated individual(s). The approval, including the date and signature of the individual(s) approving the requirements, shall be documented.
(d) Design output. Each manufacturer shall establish and maintain procedures for defining and documenting design output in terms that allow an adequate evaluation of conformance to design input requirements. Design output procedures shall contain or make reference to acceptance criteria and shall ensure that those design outputs that are essential for the proper functioning of the device are identified. Design output shall be documented, reviewed, and approved before release. The approval, including the date and signature of the individual(s) approving the output, shall be documented.
(e) Design review. Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device’s design development. The procedures shall ensure that participants at each design review include representatives of all functions concerned with the design stage being reviewed and an individual(s) who does not have direct responsibility for the design stage being reviewed, as well as any specialists needed. The results of a design review, including identification of the design, the date, and the individual(s) performing the review, shall be documented in the design history file (the DHF).
(f) Design verification. Each manufacturer shall establish and maintain procedures for verifying the device design. Design verification shall confirm that the design output meets the design input requirements. The results of the design verification, including identification of the design, method(s), the date, and the individual(s) performing the verification, shall be documented in the DHF.
(g) Design validation. Each manufacturer shall establish and maintain procedures for validating the device design. Design validation shall be performed under defined operating conditions on initial production units, lots, or batches, or their equivalents. Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions. Design validation shall include software validation and risk analysis, where appropriate. The results of the design validation, including identification of the design, method(s), the date, and the individual(s) performing the validation, shall be documented in the DHF.
(h) Design transfer. Each manufacturer shall establish and maintain procedures to ensure that the device design is correctly translated into production specifications.
(i) Design changes. Each manufacturer shall establish and maintain procedures for the identification, documentation, validation or where appropriate verification, review, and approval of design changes before their implementation.
(j) Design history file. Each manufacturer shall establish and maintain a DHF for each type of device. The DHF shall contain or reference the records necessary to demonstrate that the design was developed in accordance with the approved design plan and the requirements of this part.”
Management, creation and execution of a design history file for each type of device take experience and grit. Completing a risk analysis, or hazard analysis early in the design phase for medical devices is not only required, but is important for successful development. The FDA led in this respect, creating regulations documented in 21 CFR Section 820. Today we have standards such as ISO 13485 and ISO 14971 (risk analysis) to guide our global de-risking of medical devices. We have developed industry standard best practices for design control management including risk analysis processes that help move a device efficiently and effectively through the early stages of development. The regulations and standards are straightforward to implement, and gain complexity as company size and risk tolerance grow. Keeping control or iterating on your quality management system of industry standard best practices is important when developing a medical device and requires surveillance and dedication to keeping patients safe while not being sidetracked. Today, as our industry has evolved, device risk requirement generation has become a part of medical device design and development. These tools are useful and necessary in the development process. We have experience with risk analysis, creation of user needs, design control file creation, process validation, clinical evaluations and many other activities as identified in ISO 14971 that are required to produce a safe medical device. We can help at any stage with the development of your medical device.
Publications
Bratincsak A, Moore JW, Gulker B, Choules B, Koren L, El‐Said HG. Breaking the Limit: Mechanical Characterization of Overexpanded Balloon Expandable Stents Used in Congenital Heart Disease. Congenital Heart Disease, 2014.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
