Clinical Data Interchange Standards Consortium (CDISC)
Introduction to Implementing Study Data Standards
Importance of Study Data Standards
Exchanging data globally has become much simpler as information technologies develop. However, medical terms and the associated acronyms can quickly turn into alphabet soup (see Glossary) that may be interpreted differently depending on geographic region, therapeutic area, and personal perspective. Furthermore, use of differing data structures can make it very difficult to combine data from different sources. To achieve greater clarity and interoperability in data management, Clinical Data Interchange Standards Consortium (CDISC), a global non-profit organization, has developed standards to optimize electronic data collection, structure, and analysis. CDISC standards are free to use without licensing restrictions. These data standards also help sponsors effectively transmit the data to regulatory bodies. FDA maintains a Data Standards Catalog describing supported standards based on type of submission and reviewing center. In particular, the Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) require CDISC standard compliance, and they may refuse to file submissions that do not conform to CDISC standards. The Center for Devices and Radiological Health (CDRH) encourages the use of data standards in any submission for more efficient review. Further clarification of the requirements of standardized study data used in electronic submissions can be found in FDA’s guidance on this topic. An overview of the CDISC data processing modules is provided in the following figure (see the Glossary for definitions of abbreviations).
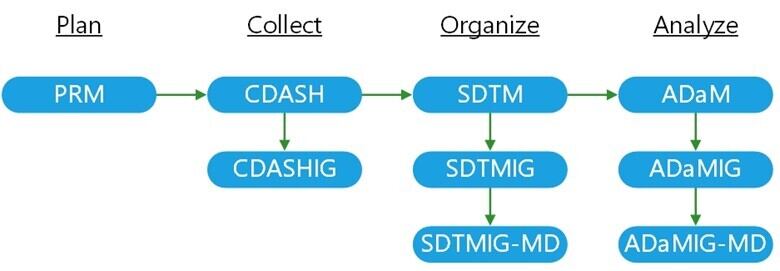
Figure 1: Diagram of CDISC standards organized by data process
Protocol Representation Model (PRM)
Due to the importance of standardized data for data exchange and regulatory submission, implementation of study data standards early in the clinical study design is strongly encouraged. The Protocol Representation Model (PRM) can aid in the design of the clinical study protocol. PRM is based on the requirements for study registration from the World Health Organization (WHO), ClinicalTrials.gov, and EudraCT in accordance with International Conference on Harmonization (ICH) guidances E6, E3, and E9. The PRM model was constructed from SDTM (Study Data Tabulation Model) elements to allow a smooth transition from study planning to actual data management.
Clinical Data Acquisition Standards Harmonization (CDASH)
For collecting data, the Clinical Data Acquisition Standards Harmonization (CDASH) and its implementation guide, CDASHIG, describe naming conventions for variables used in case report forms. The terminology dictionary contains variables that are consistent throughout the CDISC standards. Other variables can be constructed using established domain prefixes with root variables. The first part of the variable is the domain which usually corresponds to the purpose of the CRF. There are three classes of domains: interventions, events, and findings. For example, a variable for the date on a procedure CRF would have the intervention domain PR (for procedure), then another variable would describe the parameter DAT (for date), with the combined variable then being PRDAT. Variables can also be strung together as in CMDOSFRQ which codes for concomitant medication (CM) dose (DOS) frequency (FRQ). As a general rule for variable construction, numbers should not be used in the variable and it should be shorter than 12 letters long, if possible. CDASH variables are intended to map to the SDTM (see next section).
Study Data Tabulation Model (SDTM)
The Study Data Tabulation Model (SDTM) along with its implementation guide, SDTMIG, provides the framework for organizing data. The five classes of variables are identifiers, topic, timing, qualifier, and rule. Within the qualifier role, there are five subclasses of grouping, result, synonym, record, and variable. For example, MKHNDPOS codes for the hand (HND) positioning (POS) during a test in the Musculoskeletal System Findings domain (MK). There are additional terms specific for medical devices in the SDTMIG-MD. In the absence of an existing SDTM variable, then the CDASH variable can be used. However, new variables can be suggested to CDISC for addition to the SDTM.
Analysis Data Model (ADaM)
The Analysis Data Model (ADaM) is optimized for analysis of study data, with a focus on traceability. Variable naming conventions are similar to the other models listed above. According to the Analysis Data Model Implementation Guide (ADaMIG), ADaM available standard data structures include a subject-level analysis dataset (ADSL) and basic data structure (BDS). For medical device clinical studies, device-level analysis dataset (ADDL) is preferred since the identifier relates to the device and not the patient. Each structure has aspects about what needs to be included in analysis metadata reporting. Some examples include:
- Dataset information
- Dataset name
- Dataset description
- Dataset location
- Dataset structure
- Key variables of dataset
- Class of dataset
- Documentation
- Parameter identifier
- Variable name
- Variable label
- Variable type
- Display format
- Codelist / controlled terms
- Source / derivation
As you can see, effective management of electronic study data in today’s world can be complex. Contact us to discuss how to integrate study data standards throughout your clinical trial.
Glossary
- ADaM – Analysis Data Model
- ADaMIG – Analysis Data Model Implementation Guide
- ADDL – device-level analysis dataset
- ADSL – Subject-Level Analysis Dataset
- BDS – Basic Data Structure
- CDASH – Clinical Data Acquisition Standards Harmonization
- CDASHIG – Clinical Data Acquisition Standards Harmonization Implementation Guides
- CDISC – Clinical Data Interchange Standards Consortium
- PRM – Protocol Representation Model
- SDTM – Study Data Tabulation Model
- SDTMIG – Study Data Tabulation Model Implementation Guide
- SDTMIG-MD – Study Data Tabulation Model Implementation Guide for Medical Devices
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).