B1+RMS limits on MR Conditional labeling
MRI Safety
European notified bodies have recently started requiring B1+RMS limits on MR Conditional labeling. This requirement stems from IEC 60601-2-33, a standard for MRI scanners, which lists mandatory metrics that must be user selectable to the scanner operator to ensure safe scanning. Among these required metrics is B1+RMS, while SAR is optional. Like SAR, which is typically seen on MR Conditional labels for passive (no electric power) medical devices, B1+RMS is an RF exposure metric that can be used to ensure patient safety during MRI. However, the two metrics are not the same. 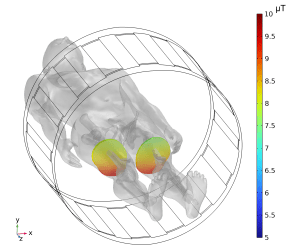
SAR is an estimate of the RF energy absorbed by the patient being scanned with units W/kg. The energy absorbed is dependent on patient habitus (e.g., size, body composition) and the anatomical location being scanned. Therefore, since SAR is dependent on several variables that the scanner cannot control, it is typically overestimated to provide conservative safety conditions.
B1+RMS is a measure of the average RF magnetic field (B1+) that is generated by the RF transmit coil during scanning and used to positively rotate the protons used for imaging, with units µT. Unlike SAR, B1+RMS is not an estimated value, but instead can be directly calculated by an MRI scanner based on the pulse sequence being used and its accompanying parameters. B1+RMS is patient independent, allowing any scanning sequence that has a B1+RMS within a limit listed on an MR Conditional label to be applicable for any patient. This, along with its ability to be directly calculated by the scanner using a standardized equation, allows B1+RMS to potentially provide safer labeling that is not unnecessarily restrictive as compared to SAR labeling.
At MED Institute, we have the ability to determine appropriate B1+RMS limits for MR Conditional labeling using computational modeling and simulation. This capability, along with the potential benefits of B1+RMS labeling, is highlighted in an abstract titled “Can B1+RMS help prevent overly restrictive MR Conditional labels for medical devices that are currently based on SAR?” that MED Institute will be presenting this Wednesday at the International Society for Magnetic Resonance in Medicine annual meeting in Singapore.
Contact us today to learn more about how we can provide MR Conditional labeling with B1+RMS limits for your devices 855.463.1633.
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).

