Leveraging Surface Characterization
Emerging Trends in Biocompatibility Tests
Finding the least burdensome pathway to address biocompatibility requirements can be a challenge. Coupling our engineering and testing lab with a certified toxicologist has allowed us to find the safest and most efficient pathway to many surface property answers in relation to biocompatibility. We will lay the foundation for answering biocompatibility questions in this post then follow on in a subsequent post with some case studies that may help.
Understanding the surface properties of medical devices is one crucial safety attribute to biological evaluation. For many devices, chemical analysis alone is insufficient to address the safety of the device in its final, finished form, as many of the tests do not use the final form in the actual biocompatibility test. Ensuring that the surface properties of devices are safe and do not elicit any undesirable biological effects is paramount for their clinical and market success. Whether it is a new medical device or to establish a similar device (one that has equivalent properties to the clinically established device in terms of biological safety and clinical performance), a thorough understanding of the surface properties is a must. The key aspects of the surface properties are physico-chemical, morphologic, and topographic properties and presence of particulates, among others.
In recent years, there has been a growing interest among manufacturers in supplementing or complementing biological testing methods for surface characterization. Finding potential downstream clinical risk events early through bench testing is a safer and less expensive de-risking activity. For example, when using a new cleaning agent in a manufacturing step, the implantation biocompatibility endpoint can be addressed through evidence from optical profilometry to demonstrate that the surface characteristics are unchanged due to the proposed cleaning step. In the same way, a comparative surface roughness evaluation may be sufficient to address a change in material in a stent (but not the geometry) and obviate the need for thrombogenicity testing. Traditional biological tests may be appropriate for characterizing a completely novel device; however, they may be time-consuming, resource-intensive, and may not be necessary to understand a minor manufacturing change.

What is Surface Characterization?
Surface Characterization refers to the comprehensive analysis of a material’s surface properties including size, shape, physico-chemical characteristics, morphology, topography, roughness, surface energy, and associated forces (mechanical, thermal, or electromagnetic) exerted by the surfaces. These properties determine how the surface interacts with its environment, particularly with biological tissues and fluids.
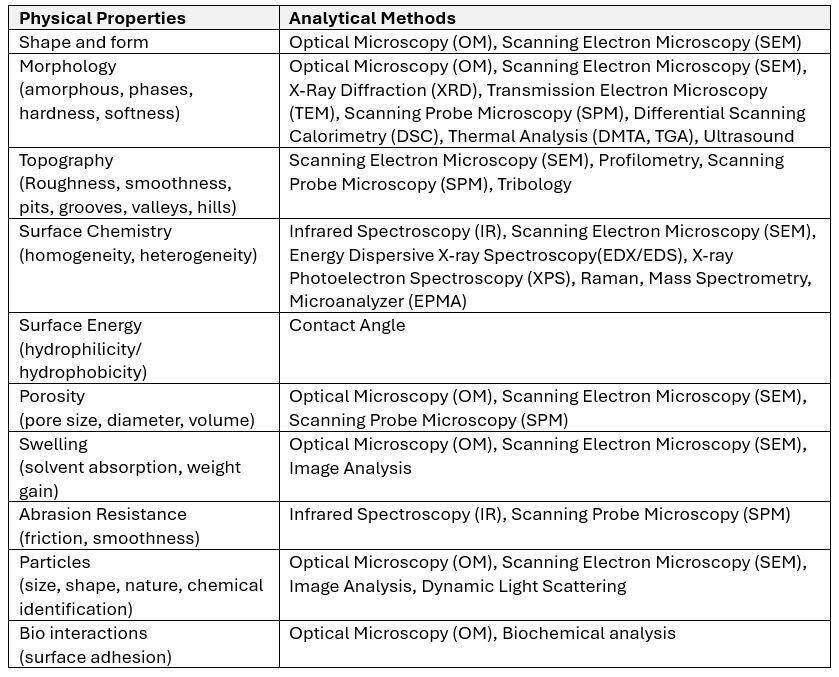
What Analytical Tests are Recommended by the Standards?
Surface topography and roughness may significantly affect biological behavior and tissue responses and can be measured using various techniques such as scanning electron microscopy (SEM) and atomic force microscopy (AFM). Techniques such as Infrared Spectroscopy, Energy Dispersive-X-ray photoelectron spectroscopy (EDX/EDS/XPS) and Electron Spray for Chemical Analysis (ESCA) are often employed to study chemical characteristics of a surface.
The analytical methods should be selected based on the physical (solid, liquid, gel, composite, animal tissues) and chemical properties (oxidation, hydrophobicity) of the surface materials of the final, finished device. Due to the diversity of medical devices, it is recognized that not all of the parameters identified for a material will be relevant for all/some of the medical device and its clinical uses. As noted in ISO 10993-1:2018, the extent of characterization which should be considered is determined by the invasiveness and duration of clinical exposure during the intended use. The obtained surface characteristics should be useful for the risk assessment of the biological safety of the device. Manufacturers should document the level of characterization performed on their medical device and its component level, including materials appropriate to its clinical application.
The ISO 10993-19 standard provides a list of potential analytical methods that are helpful to assess the typical surface properties of the materials, including polymers, metals, alloys, ceramics, and natural macromolecules.
Surface characterization is essential for understanding and optimizing the interactions between biomaterials and biological tissues. By analyzing and controlling surface properties, researchers and engineers can design materials that promote desired biological responses and minimize adverse reactions.
MED Capabilities
Coupled with our in-house toxicologist, MED Institute offers a comprehensive suite of surface characterization services that are essential for the development and validation of medical devices. MED maintains an ISO/IEC 17025:2017 accreditation and can produce validations for many surface characterization methods. We have extensive experience in assessing the surface roughness of cardiovascular implants, analyzing the wear resistance of orthopedic implants, measuring fatigue, corrosion, and particulates of catheters or stents, and understanding the biological adhesion of drug delivery systems. Whether it is a complex or simple surface characteristic, MED Institute is equipped to provide detailed and reliable analysis to support innovation in the medical device industry. Testing services are not limited to those properties that are relevant to biological evaluation or standards (ISO or ASTM) but also can be customized to evaluate any physical properties of the materials or device. MED has consistently demonstrated its ability to secure medical device approvals, be it through the 510(k) premarket notification (PMN) or De Novo process or any other regulatory pathways in any part of the world.
Contact us today to learn more about how we can partner with you.
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).

