MRI Heating Simulation
Computational Modeling and Simulation
Regulatory authorities are raising the bar for medical device manufacturers to evaluate the MRI safety of their devices. Product line combinations can be quite expansive, but MED Institute can help medical device manufacturers using computational modeling and simulation in lieu of physical testing to affordably obtain MRI safety labeling. Our analysts use scientific rationale, fundamental engineering knowledge and simulation to narrow extensive product families with thousands of configurations to one worst-case device for physical testing. CM&S is also used to determine the locations for temperature probes for physical testing. MED Institute has a successful track record with MRI safety evaluations submitted to regulatory authorities around the world.
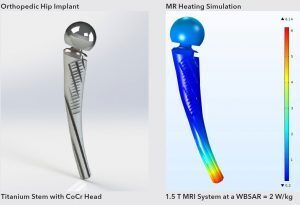
MRI Heating Simulation of Hip Implant
Virtual Human Anatomies
MED Institute uses virtual human anatomies to provide understanding on biomechanical interactions, computational fluid dynamics, and RF-induced heating during MRI. We can leverage the software to investigate numerous device designs, device positioning and boundary conditions quickly and efficiently. Additionally, virtual human anatomies allow for the investigation of clinically relevant anatomical and physiological conditions, such as perfusion and vascular flow.

OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
