Active Implantable Medical Devices (AIMDs)
ISO/TS 10974
Active implantable medical devices (AIMDs) are implanted devices that depend on an external energy source, usually electrical, for their functionality. Common examples of AIMDs include pacemakers, neurostimulators, and implantable defibrillators.
Compared to passive implantable medical devices, AIMDs pose additional risks to patients in the magnetic resonance imaging (MRI) environment, such as unintended device stimulation and device malfunction. Additionally, evaluation of radiofrequency-induced heating of AIMDs with long metallic leads requires a more rigorous approach than passive implants because of the lead path-dependent nature of heating.
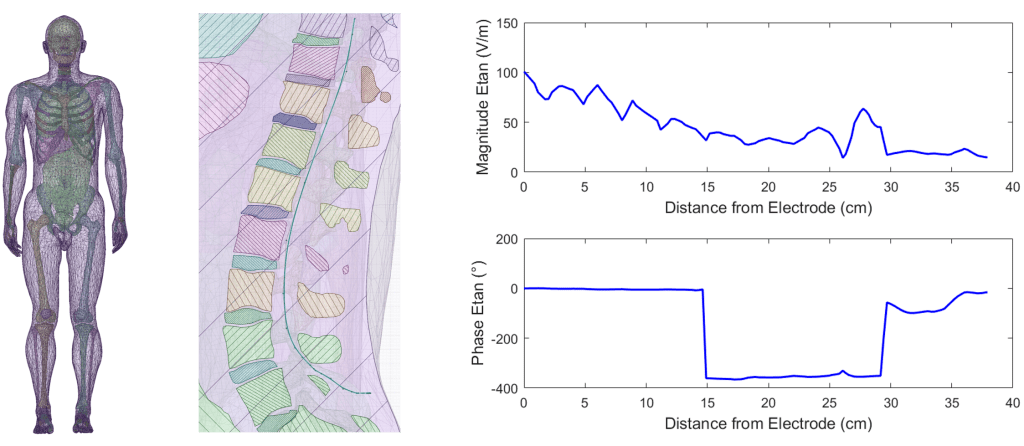
Our team of engineers and scientists can evaluate all potential hazards of AIMDs according to ISO/TS 10974, provide MRI safety labeling to be used in the instructions for use (IFU), and help support regulatory submissions.
ISO/TS 10974 Evaluations:
Hazard | Evaluation |
Heating | RF-induced heating, Gradient-induced heating |
Vibration | Gradient-induced vibration |
Force | Magnetically induced displacement force |
Torque | Magnetically induced torque |
Unintended Stimulation | Gradient-induced lead voltage, RF injection testing to test for rectification |
Device Malfunction | B0-induced device malfunction, RF injection testing to test for malfunction and rectification, Gradient-induced device malfunction, Combined fields |
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
