-

Completing clinical study enrollment and what’s next
Great job on completing enrollment! Reaching this major milestone is a big accomplishment as it marks the...
-

Understanding the Key Players in a Clinical Research Trial
In a clinical research trial, or study, various roles are crucial to ensuring the study’s success and...
-

Artificial Intelligence in Clinical Studies
Artificial Intelligence (AI) is a growing field of interest for many industries, including those of medical devices...
-

Selecting the Right Electronic Data Capture (EDC)
What is EDC? EDC stands for electronic data capture. This usually refers to the processes of an...
-

Phases and Stages of Clinical Trials
Clinical research for pharmaceuticals and devices follows the same general path. The first trial is a small...
-

Decentralized Clinical Trials
Decentralized clinical trials (DCTs) are becoming increasingly popular, with the increased use of electronic systems and novel...
-

The Value of a Clinical Events Committee (CEC)
What is a CEC? In clinical trials that include a high-risk patient demographic, a clinical events committee...
-

The Power of Post-Market Clinical Data
For medical devices, ensuring safety and efficacy doesn’t stop once a product hits the market. Post-market clinical...
-

Clinical Site Selection: Navigating the Complexities of Clinical Trial Success
Selection of an investigational site for a clinical trial is critical to success. Throughout a clinical trial,...
-

Clinical trial monitoring
Monitoring Clinical trial monitoring is a constantly evolving field, now encompassing various approaches that work together to...
-

On the Record: Compliance with 21 CFR Part 11 in Clinical Trials
What is 21 CFR Part 11? The United States Code of Federal Regulations (CFR) includes regulations for...
-
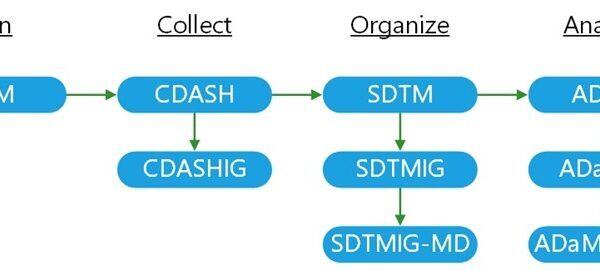
An Introduction to Implementing CDISC Study Data Standards
Importance of Study Data Standards Exchanging data globally has become much simpler as information technologies develop. However,...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
