-

B1+RMS limits on MR Conditional labeling
European notified bodies have recently started requiring B1+RMS limits on MR Conditional labeling. This requirement stems from...
-

Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions
FDA recently issued a Letter to Industry regarding medical device submissions that contain fraudulent and unreliable third-party...
-
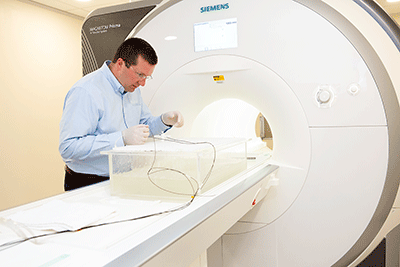
Expanding MR Conditional labeling options using new laboratory equipment
We recently added new equipment to our MRI safety evaluations laboratory that can be used to provide...
-

RF-Induced Heating of AIMDs
Next month, we will be presenting at the National Institutes of Health (NIH) as part of the...
-
FDA grants MED Institute MDDT (Medical Device Development Tool) qualification
West Lafayette, IN: MED Institute obtained FDA qualification of their MDDT (Medical Device Development Tool) for virtual...
-
MRI Safety Labeling of Medical Devices: Change Is on the Horizon
If you missed us at RAPS Convergence 2021, you can reach out to our team and we...
-
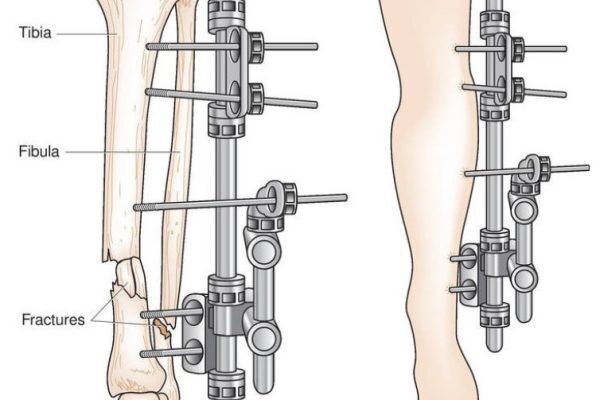
External Fixation Devices
An external fixation device refers to a device that provides stability and alignment for fractured bones and...
-

EU MDR Extension
Although the implementation of EU MDR has been postponed for up to one year, it will be...
-

Exploring the EU MDR’s Impact on ‘Legacy’ Medical Devices
Devices already legally on the market under the European Union’s (EU) Medical Devices Directive (MDD) or the...
-
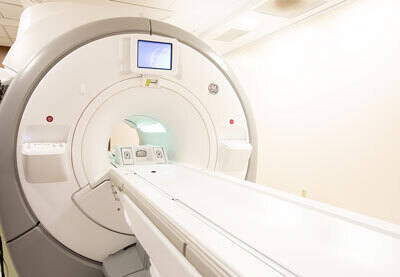
Top 10 Challenges for MRI Safety Evaluation
There are several safety concerns for patients with metallic implants who require MRI, including magnetic forces, torques,...
-
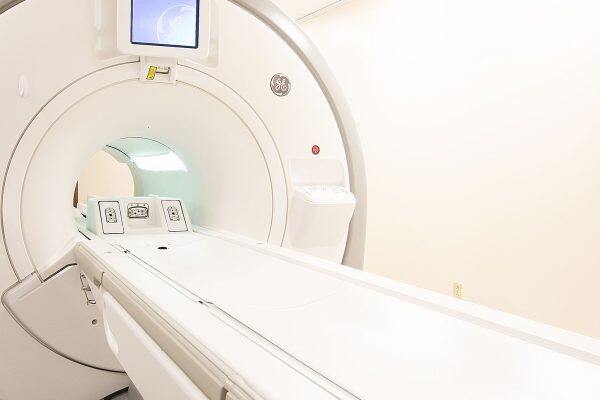
MRI Safety of Electrically Active Devices
We have recently expanded our capabilities to include MRI safety evaluation of electrically active devices to our...
-

MDR – European Medical Device Regulation
The European Medical Device Regulation (MDR) is a new set of regulations that will become effective May...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
