External Fixation Devices
We can assess the MRI safety of your external fixation device.
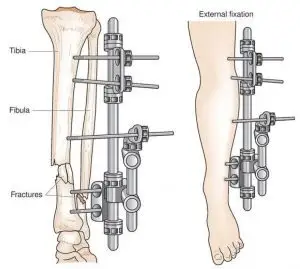
An external fixation device refers to a device that provides stability and alignment for fractured bones and can be adjusted externally. The device is partially implanted in tissue and bone but a portion of the device remains external. These devices may consist of external elements (bars, rods), connector elements (clamps), and anchorage elements (pins, screws, wires). These devices are commonly used to heal bone fractures in the forearms, legs, hands, and feet following traumatic injury.
https://medical-dictionary.thefreedictionary.com/external+fixator
External fixation devices behave differently in the magnetic resonance environment than solely internal implants. These devices can show unique characteristics with respect to radiofrequency (RF)-induced heating. For example, the conductivity of the external components in air can cause a high temperature rise in the anchorage elements residing in the bone and surrounding tissue. To prevent discomfort and potential injury to the patient, external fixation devices should be evaluated for MRI safety and subsequently labeled appropriately.
We are PJLA accredited to ISO/IEC 17025:2017, BSI certified to ISO 13485:2016, and audited to ISO 14155:2011. We can evaluate your external fixation device for MRI safety and provide labeling for your device. We can test for magnetically induced force and torque, MR image artifact, and radiofrequency-induced heating. External fixation devices can be highly customizable to fit the needs of the patient resulting in many possible configurations. Therefore, prior to physical testing, we use computational modeling and simulation (CM&S) to determine the worst-case construct for RF induced heating. Important parameters to consider for external devices are anchorage insertion depth, anchorage spacing, and external element material conductivity.
Contact us today for more information about the unique considerations of external fixation devices and how we can partner with you. medinstitute.com | 855.463.1633 | 765.463.1633 | askmed@medinstitute.com
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).

