-

A Quick Guide to the EU IVDR
The In Vitro Diagnostic Directive (EU) 98/79/EC (IVDD) was introduced over two decades ago to establish the...
-

The Growth of Digital Health Technology for Patient Reported Outcomes in Clinical Trials
An update to our blog about how digital health technology is optimizing the clinical research industry. Introduction...
-

B1+RMS limits on MR Conditional labeling
European notified bodies have recently started requiring B1+RMS limits on MR Conditional labeling. This requirement stems from...
-

Navigating Biocompatibility: An Introduction to ISO 10993 Series of Standards
Medical device manufacturers should always refer to ISO 10993 Series of Standards in addition to region-specific regulatory...
-

Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions
FDA recently issued a Letter to Industry regarding medical device submissions that contain fraudulent and unreliable third-party...
-

3 Steps to MDR/IVDR Compliant Systematic Literature Review
A systematic literature review is a method to collect, identify, and synthesize literature as objectively as possible...
-
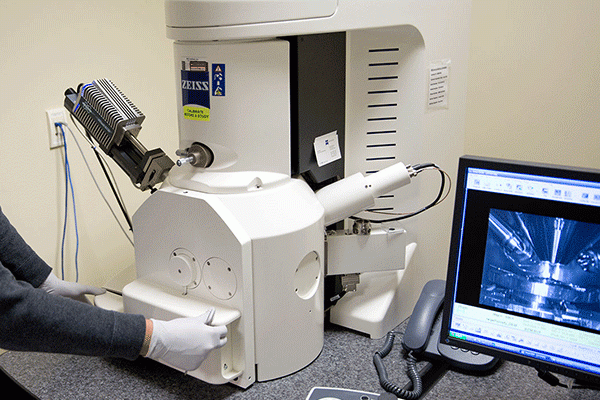
Failure Analysis Testing
Medical device innovations have greatly improved patient care and outcomes over the past few decades. As we...
-

FDA’s New Guidance on Nitrosamine and Nitrosamine- Drug Substance Related Impurities and Recommended Acceptable Intake Limits
The FDA has recently published a Recommended Acceptable Limit guidance document for nitrosamine impurities in drug products....
-
Navigating the Complex World of PFAS Regulation for Medical Devices
Per- and poly-fluoroalkyl substances (PFAS) are a group of fluorinated compounds characterized by carbon-fluorine bonds. While some...
-

Clinical trial monitoring
Monitoring Clinical trial monitoring is a constantly evolving field, now encompassing various approaches that work together to...
-

An important downside to eSTAR
If you are keeping abreast of FDA’s medical device efforts, then you know that as of October...
-

Creating a Conference Presentation: All You Need is an Hourglass
Creating an easy to follow, interesting, conference presentation is always the goal of conference attendees, but quite...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
