-

What is a Clinical Evaluation Report (CER)?
A Clinical Evaluation Report (CER) is a detailed summary of the evaluation of information pertaining to clinical...
-

The Value of an ISO/IEC 17025:2017 Accreditation
In 2004, we decided to institute an ISO/IEC 17025 quality management system designed specifically for calibration and...
-

Patient Engagement in Clinical Trials: Patient, Industry, and Clinical Investigator Perspectives
The Medical Device Innovation Consortium (MDIC) Science for Patient Input initiative has been working to promote patient-centered...
-
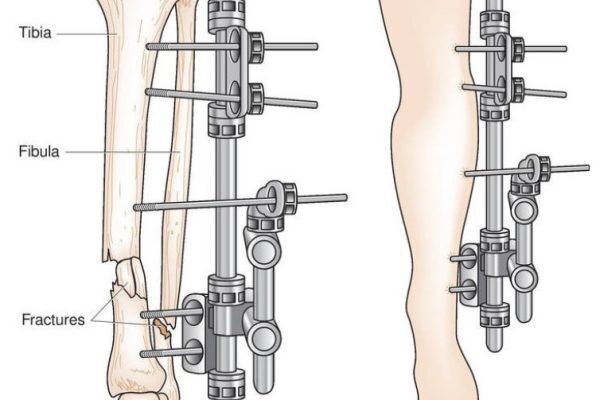
External Fixation Devices
An external fixation device refers to a device that provides stability and alignment for fractured bones and...
-

How does an Early Feasibility Study differ from a Pivotal Study?
An Early Feasibility Study (EFS) is performed early in the device development process to evaluate the device...
-

Quality System Remediation and Root Cause Analysis
Successful quality system remediation relies on understanding why problems arose, generally referred to as “root cause analysis.” ...
-

Hybrid Clinical Trials in the Age of COVID-19 and Beyond
We were recently invited by the orthopedic market intelligence firm ORTHOWORLD® to describe our experiences with clinical...
-

Using Patient Preference Information (PPI) for Patient-Centered Medical Device Development
There is much interest in engaging patients in the medical device development process, at all stages of...
-

Remediation, Regulatory Compliance, and Company Culture
FDA Warning Letters are devastating – a Quality Manager’s worst nightmare – a major shock to any...
-

EU MDR Extension
Although the implementation of EU MDR has been postponed for up to one year, it will be...
-

Top 3 Challenges for Particulate Matter Evaluation
There are several safety concerns (risks) for patients who require a minimally invasive procedure. One of the...
-

Exploring the EU MDR’s Impact on ‘Legacy’ Medical Devices
Devices already legally on the market under the European Union’s (EU) Medical Devices Directive (MDD) or the...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
