Phases and Stages of Clinical Trials
Clinical Trial Services
Clinical research for pharmaceuticals and devices follows the same general path. The first trial is a small pilot study to gain insight on how a larger study should be designed based on preliminary safety and efficacy data. The next trials focus on safety then efficacy of the investigational product with increasing numbers of subjects. Once enough data are collected to support the safety and efficacy, they can be submitted to a regulatory body for market approval. After approval, further studies can reveal more long-term trends in a larger real-world population.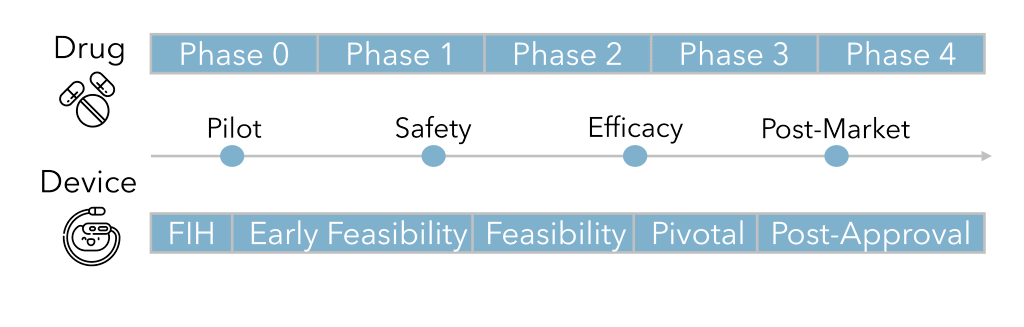
Pharmaceutical studies
Pharmaceutical clinical trials consist of five “phases”. Phase 0, also called “early Phase 1” or “exploratory investigational new drug (IND) study ”, is a pilot study of 10-15 people to test a subtherapeutic small dose over the course of a week to determine pharmacodynamics and pharmacokinetics. This phase aims at reducing the number of clinical trial failures in later phases and increasing patient safety by providing a first-in-human look (usually in healthy volunteers) before scaling-up to a bigger study with higher doses. The data from Phase 0 can shape study design and patient selection of larger trials.
The next step, Phase 1, is to evaluate the safety of the drug in 20-80 healthy volunteers or cancer patients over the course of several months. Safety testing includes determining the maximum tolerable dose and identifying side effects. Also in this phase, research will explore the pharmacodynamics and pharmacokinetics to gather information about the mechanism of action and drug metabolism.
Phase 2 continues to studies the safety of the drug, but in several hundred patients with the target disease or condition, lasting from several months to two years. In addition to learning about the efficacy of improving the disease or condition, investigators can determine the therapeutic dose level and frequency.
In Phase 3 or “pivotal” studies , several hundreds to a few thousand patients will be studied for 1-4 years. Due to the scale and duration, long-term and rare side effects can be observed. Clinical efficacy of the investigational pharmaceutical is compared to standard of care or other pharmaceuticals on the market. For this reason, studies are likely to be double-blinded and randomized.
The final phase, Phase 4 is conducted after market approval to monitor the safety and efficacy of the drug long-term in the general “real-world” patient population. One type of Phase 4 study is a registry which is a prospective observational study of a patient cohort or a few different patient cohorts to provide additional insight about the drug’s effect on different patient subpopulations.
Device studies
Medical device clinical studies have three major steps called “stages”. The first or pilot stage is called “early feasibility” or “first-in-human (FIH)”. The pilot study will include 10-15 patients to gather preliminary safety and performance data to support its benefit/risk profile. The results during the study can be used to guide design modifications and refine study parameters. In the United States, an investigational device exemption (IDE) might be required to conduct this type of study.
Pivotal studies of hundreds of patients provide data which can be statistically analyzed to answer hypotheses about safety and effectiveness. These studies may include comparison with other devices and/or standard of care. Placebos in medical device trials are rare since the benefit/risk profile of a sham surgery (one where the surgery is performed, but a device is not implanted) may not be favorable. However, some devices may be non-invasively activated/inactivated which can provide a level of blinding and randomization through cross-over. For more information about the difference between early feasibility and pivotal studies check out our other blog post.
Similar to Phase 4 studies for drugs, the post-approval stage of medical device clinical trials occurs after the device has been approved for marketing or may be a condition of approval (for example, may be required by the FDA under Section 522 for certain PMA devices). A larger patient population is monitored for longer term safety and effectiveness. Post-market clinical data is important because it enhances patient safety, supports evidence-based medicine, and increases market transparency and accountability.
MED offers a variety of clinical trial services and is a full-service CRO. We have over 40 years of experience designing and executing clinical trials, ranging from early feasibility studies to multinational, controlled pivotal trials to post-market registries.
Contact us today to start your project discussion.
855.463.1633 | medinstitute.com
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).