What is regenerative medicine?
Regenerative medicine is an interdisciplinary field that integrates engineering and life science principles to develop and deliver therapies that heal, regrow or replace damaged or diseased cells and tissues due to aging, disease, or defects. Regenerative medicine represents the next revolution in medicine, using the body’s own machinery to heal and repair, and creates hope for previously incurable diseases. To date, the strategies for treating diseased or traumatized tissue and organs have been limited, producing outcomes that provide symptomatic relief at the expense of the loftier goal of complete repair or remission of disease. The scientific and clinical efforts with regenerative medicine go beyond symptomatic relief and disease management, generating novel and effective new therapies to aid the body in restoring and repairing itself to improve patient quality of life.
Regenerative medicine will help shape the future of healthcare by relying on the body’s innate healing ability to promote regeneration, providing faster healing and recovery time, reduced need for medications, less invasive procedures, and potentially eliminate the need for surgery.
Developing Regenerative Medicine Therapies
Navigating the product development pathway from the bench to the clinic depends on a variety of knowledge, skills, and experiences. Regenerative medicine companies are becoming more and more reliant on contract research organizations (CROs) to augment their own scientific expertise, reduce development expenses, quicken time to market, and deal with the complexities of cell, gene, and tissue-based therapies.
Product development in the regenerative medicine space demands comprehensive, customized solutions. CROs that offer integrated services can be the one-stop shop that optimizes efficiency thorough the entire therapy development process. Drawing on teams with extensive scientific, regulatory, clinical and commercial experience enables us to effectively address client critical needs, meet project expectations, and drive innovation with the candidate therapeutic product.
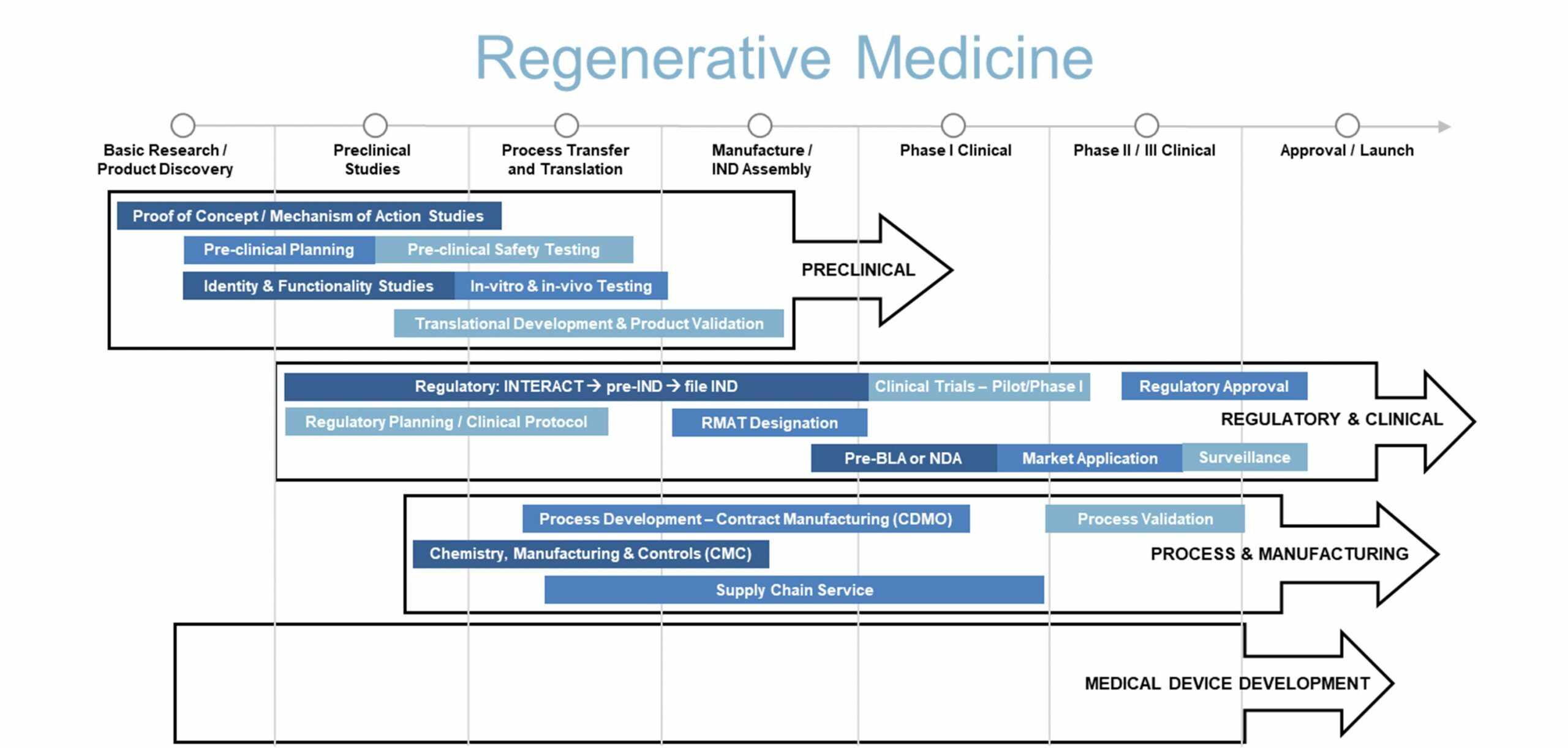
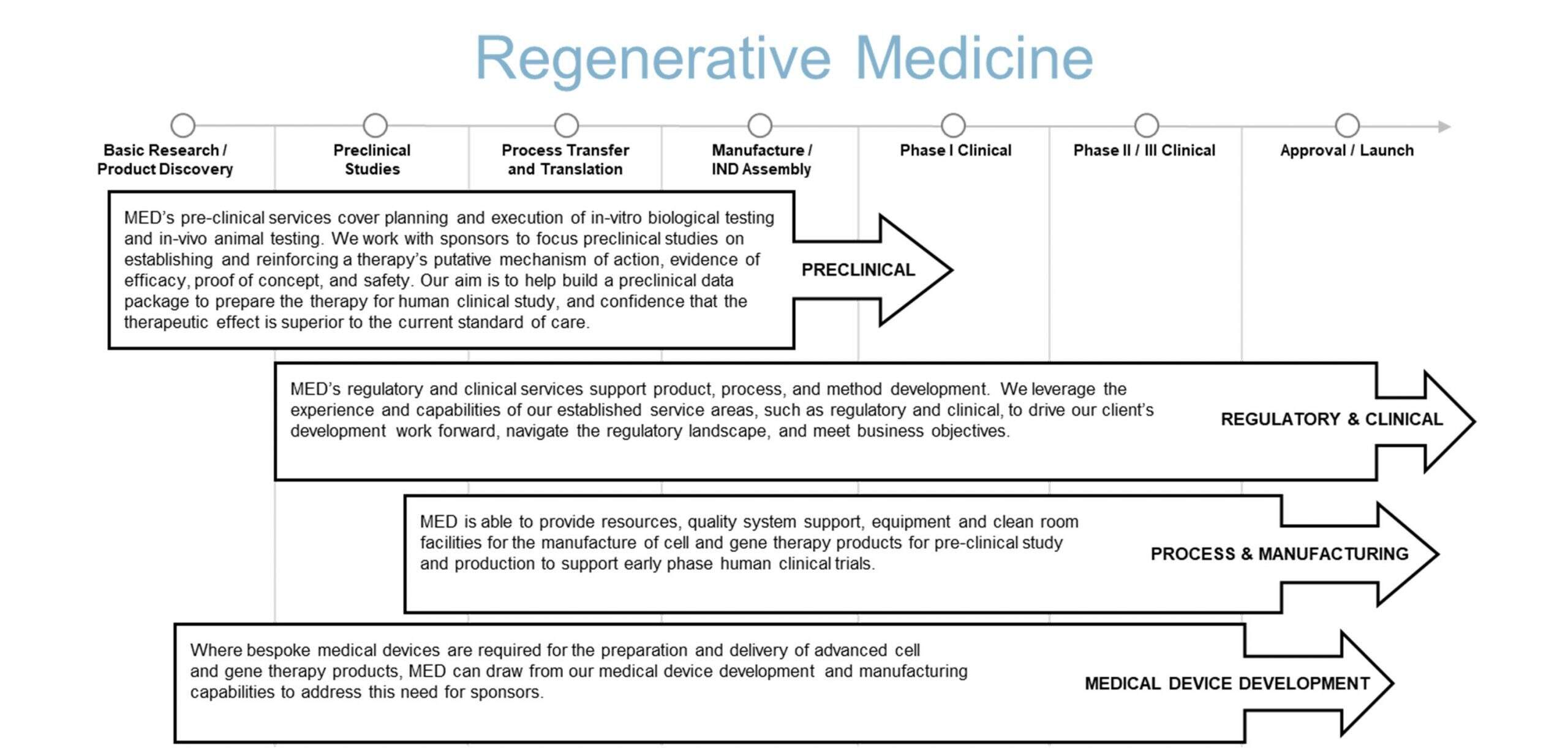
The following diagram illustrates our conceptualization of the product development pathway for a regenerative medicine product. We aim to form effective partnerships with clients, leveraging our broad based services to accelerate product development.
Our regenerative medicine services primarily focus on the early stages of product development, helping sponsors move a product from early concept to a successful IND submission and then to early clinical evaluation. Our development services aim to reduce time, cost, complexity, and ultimately risk for sponsors at each project milestone or phase.
For more information on our regenerative medicine services and to get your project started, please contact us today 855.463.1633 | askmed@medinstitute.com | medinstitute.com.
Also of interest:
Canestrari E, Steidinger HR, McSwain B, Charlebois SJ, Dann CT. Human Platelet Lysate Media Supplement Supports Lentiviral Transduction and Expansion of Human T Lymphocytes While Maintaining Memory Phenotype. Journal of Immunology Research, 2019.
Thompson S, Klarer A, Smith D, Charlebois S, Steidinger H, Taylor A. Improving the quality cell yield of T-cell immunotherapies through selective pressures imparted by culture media supplements. Cell & Gene Therapy Insights 2020; 6(2), 287–294.
Charlebois SJ, Canestrari E, Harris S. Characterization of a pathogen reduced human platelet lysate. Cytotherapy, Volume 20, Issue 5, Page S61, May 2018.
Canestrari E, Charlebois SJ, Harris S. Human platelet lysate as a media supplement for ex vivo expansion of immune cells. Cytotherapy, Volume 20, Issue 5, Page S61, May 2018.
Charlebois SJ, Ramachandran N, Hiles MC, McRoy W, Poderycki M, Steidinger H, Kuske J. Design verification and enhanced risk mitigation tests for cytocompatibility evaluation of cell delivery devices. Cytotherapy, Volume 16, Issue 4, Page S48, April 2014.
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).