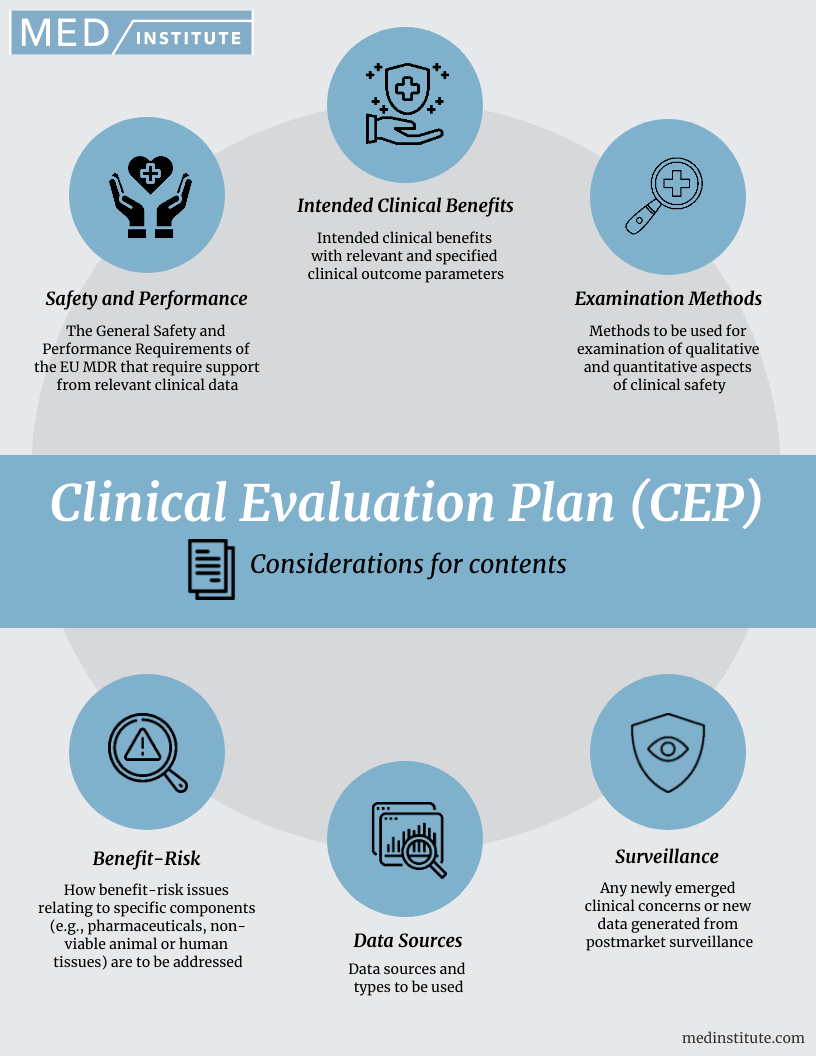
Clinical Evaluation Plan (CEP): 6 Content Considerations
The clinical evaluation plan (CEP) defines the methods for creating and updating the clinical evaluation report (CER). Your clinical evaluation plan will define the extent of information to gather and the systematic approach to be taken; the amount of data required will be influenced by the nature of the device, its stage in the life cycle and its safety record. The CEP is the first document to be prepared during the clinical evaluation process.
We have the tools and the experience to help you with your medical device project needs, from concept to commercialization. We are qualified clinical evidence reviewers and have the experience and appropriate skill set to define the scope of your clinical evaluation and expertly develop your CEP to help meet the current EU MDR.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
