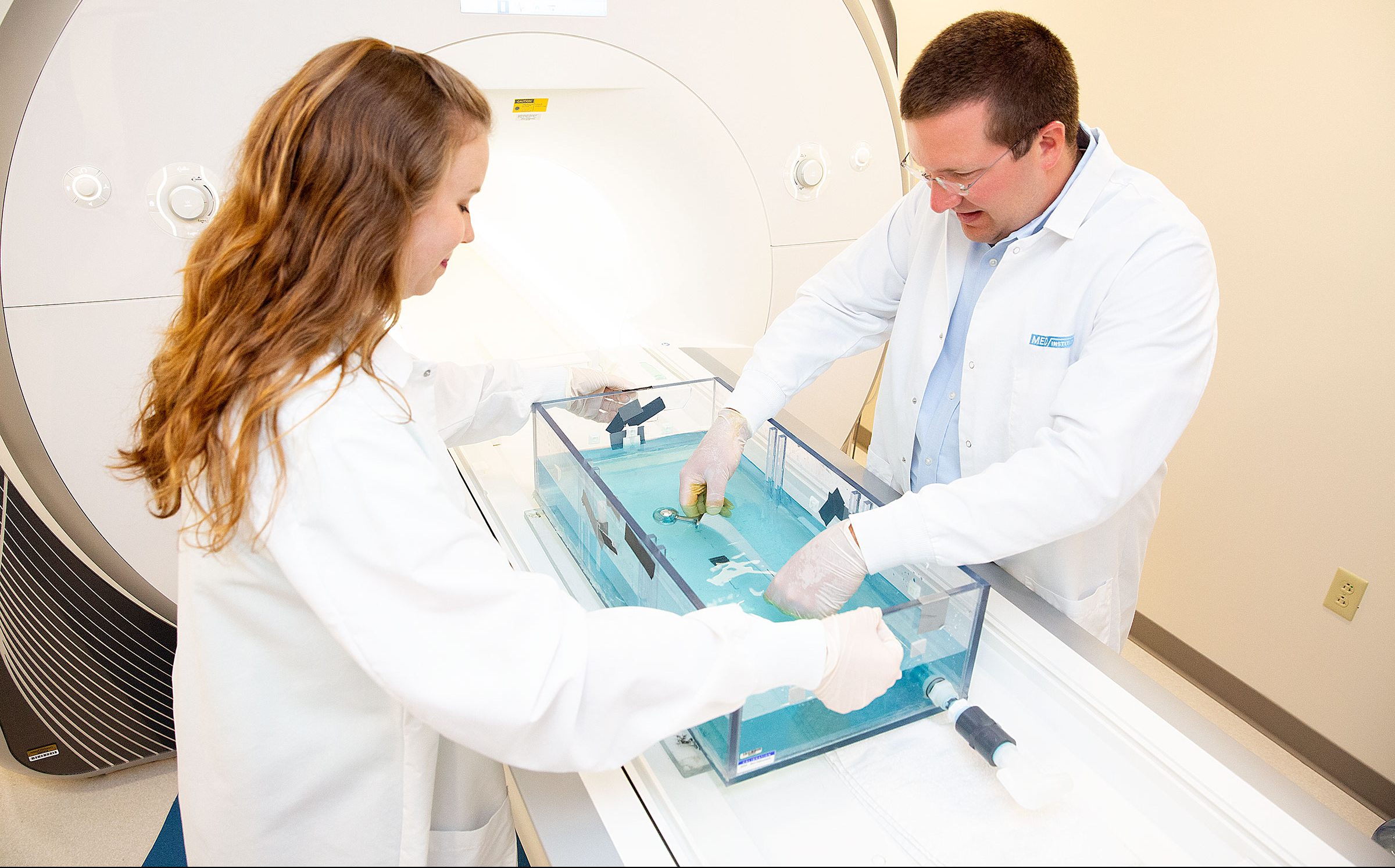
When you need a team with broad experience and knowledge who really wants to get your medical product idea to the market where it can help patients, we are ready to collaborate. Contact us to learn more and to discuss what we can do to help guide your project through the medical device product lifecycle.
Latest News
The latest news and thought leadership from MED Institute.
-

Navigating Biocompatibility: An Introduction to ISO 10993 Series of Standards
April 2, 2024Medical device manufacturers should always refer to ISO 10993 Series of Standards in addition to region-specific regulatory...
-

Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions
March 21, 2024FDA recently issued a Letter to Industry regarding medical device submissions that contain fraudulent and unreliable third-party...
-

3 Steps to MDR/IVDR Compliant Systematic Literature Review
March 18, 2024A systematic literature review is a method to collect, identify, and synthesize literature as objectively as possible...
OUR COMMITTMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in the last two quarters, we received ratings of 4.76/5 points (10).