-

Key Takeaways from the FDA Draft Guidance on Chemical Analysis for Biocompatibility Assessment – Part 1 of 2
Within the last five years, there has been significant advancement in chemical analysis and toxicological risk assessment...
-

Leveraging Surface Characterization: Emerging Trends in Biocompatibility Tests
Finding the least burdensome pathway to address biocompatibility requirements can be a challenge. Coupling our engineering and...
-

Fraudulent and Unreliable Laboratory Testing Data in Premarket Submissions
FDA recently issued a Letter to Industry regarding medical device submissions that contain fraudulent and unreliable third-party...
-
Failure Analysis Testing
Medical device innovations have greatly improved patient care and outcomes over the past few decades. As we...
-

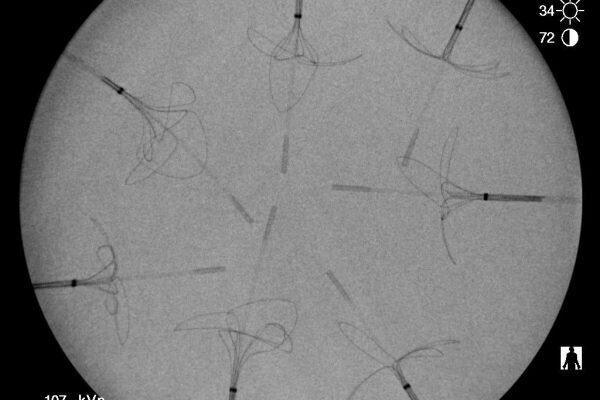
Radiopacity evaluation of a medical device
During the summer of 2022, we expanded into a new space to better serve our customers. This...
-

FDA grants MED Institute Accreditation Scheme for Conformity Assessment (ASCA)
FOR IMMEDIATE RELEASE: August 18, 2021 Colleen Tennyson MED Institute 855.463.1633 ctennyson@medinstitute.com MED Institute Receives Accreditation through...
-

Particulate Matter Spiking and Recovery
In an earlier blog post, we identified the top three challenges for performing particulate testing: acceptance criteria...
-

Top 3 Challenges for Particulate Matter Evaluation
There are several safety concerns (risks) for patients who require a minimally invasive procedure. One of the...
-
Radiopacity Evaluation and The Top 10 Challenges
The ability to visualize a minimally invasive medical device or product during use is an important consideration...
-

Pushing the limits of medical device design
Our multi-disciplinary team has extensive experience in medical device research and development, including but not limited to...
-

White Paper: Radial Force
Radial Force Testing Radial force testing is used to determine the stiffness (hoop strength) of a medical...
-

The Goals of Test Method Validation
Test method validation is a documented process that is used to confirm that the procedure to be...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
