What is a Clinical Evaluation Report (CER)?
A Clinical Evaluation Report (CER) is a detailed summary of the evaluation of information pertaining to clinical use of a medical device. All sources of clinical data are considered, as well as certain non-clinical testing data that may affect the benefit-risk analysis for the device. Taken together, the information included in the CER provides a thorough understanding of the safety and performance of a device and provides a basis to make a determination whether the device has an acceptable benefit-risk ratio for its intended purpose.
Steps to create a clinical evaluation report
The initial step is defining the scope of the clinical evaluation and creating a plan. The clinical evaluation plan (CEP) describes what devices will be the subject of the CER including device sizes, the intended purpose, target patient populations, medical indications and the clinical benefit to patients. The next step is to identify the available data on the device and determine a literature review strategy. 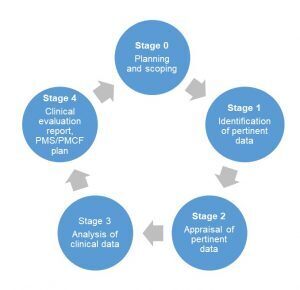
Because the contents of a CER vary according to the nature and regulatory history of the medical device, it can be challenging to create a universal template to structure all of a company’s CERs. To ensure a CER is comprehensive the following general contents should be considered for inclusion:
- Description of device and its use, along with regulatory history and device classification
- Summary of the clinical background for your device and its intended purpose(s)
- Systematic, documented searches of available clinical literature and clinical practice guidelines to create a thorough review and evaluation of the relevant state of the art, ultimately requiring qualified medical review
- Demonstration of equivalence to other device(s), based on technical, biological, and clinical characteristics, if applicable
- Summary of nonclinical testing data
- Summary of sales, complaints, clinical data held by the manufacturer, and other post-market surveillance data
- Systematic, documented searches of published clinical study reports and other clinical data to create a comprehensive review of clinical experience of your device (or equivalent), again requiring qualified medical review
- Summary of device usability data
- Appraisal of the relevance, applicability, quality, and significance of the available data
- Explanation of the methods and parameters used to assess clinical benefits, safety, and the benefit-risk ratio
- Summary of benefit-risk determination
 It is critical for medical device companies to ensure that their Clinical Evaluation Report is well prepared and maintained. While it may seem a cumbersome regulatory activity, the ultimate goal is the advancement of patient safety. If an outside vendor is helping to complete a CER, the device manufacturer will still need to compile and share as much of the above information as possible, to ensure a quality CER and a smooth writing process.
It is critical for medical device companies to ensure that their Clinical Evaluation Report is well prepared and maintained. While it may seem a cumbersome regulatory activity, the ultimate goal is the advancement of patient safety. If an outside vendor is helping to complete a CER, the device manufacturer will still need to compile and share as much of the above information as possible, to ensure a quality CER and a smooth writing process.
We have the experience and expertise to efficiently develop your CER to help meet the current EU MDR. Please contact us today to get started on your path to compliance 855.463.1633 | askmed@medinstitute.com | medinstitute.com.
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).

