What Should You Do When a Testing Standard Doesn’t Exist?
Part 2: Working through Standards Organizations
In part one of this series, we discussed how medical device technology can frequently outpace the development of standardized testing methods. One potential solution to this dilemma is to work with the standard organizations to modify an existing standardized method or to publish a new test method.
 Standards committees are typically very receptive to suggestions that might improve an existing standard.
Standards committees are typically very receptive to suggestions that might improve an existing standard.
The modification of an existing standardized method can be of great benefit to your company as well as to the industry as a whole. Revisions can occur as often as necessary and can reflect the rapid changes in technology.
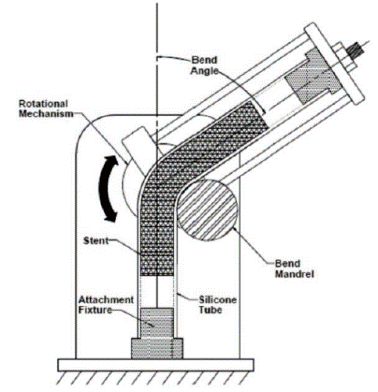 Working together with the American Society for Testing and Materials (ASTM), we helped to publish a new guide for the axial, bending, and torsional durability testing of vascular stents. Each company involved was able to incorporate their own validated methods into this new standard.
Working together with the American Society for Testing and Materials (ASTM), we helped to publish a new guide for the axial, bending, and torsional durability testing of vascular stents. Each company involved was able to incorporate their own validated methods into this new standard.
Helping to develop new standards can be a time consuming process, but it allows you to collaborate with others in the industry that are struggling with the same issues and can often be an opportunity to build industry synergy and world-wide acceptance of universal design criteria.
In the next part of this series, we’ll look at more options for establishing a new test method.
For more information, please visit our website at http://www.medinstitute.com
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent in 2024, we received ratings of 4.98/5 points (13).
