-
Failure Analysis Testing
Medical device innovations have greatly improved patient care and outcomes over the past few decades. As we...
-

Radiopacity evaluation of a medical device
During the summer of 2022, we expanded into a new space to better serve our customers. This...
-

Particulate Matter Spiking and Recovery
In an earlier blog post, we identified the top three challenges for performing particulate testing: acceptance criteria...
-

Top 3 Challenges for Particulate Matter Evaluation
There are several safety concerns (risks) for patients who require a minimally invasive procedure. One of the...
-
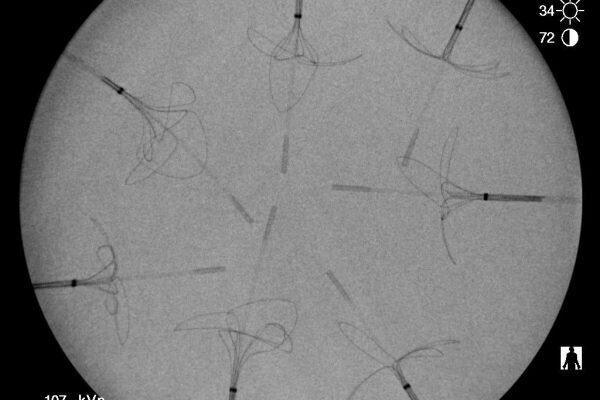
Radiopacity Evaluation and The Top 10 Challenges
The ability to visualize a minimally invasive medical device or product during use is an important consideration...
-

Early Feasibility Studies (EFS) Program
The idea of the Early Feasibility Studies (EFS) program was first published in 2011 as a FDA...
-

Pushing the limits of medical device design
Our multi-disciplinary team has extensive experience in medical device research and development, including but not limited to...
-

Test Method Development and Validation
The requirements associated with medical device safety testing continue to increase, which has raised the bar for...
-

Avoid these pitfalls in early design phases
Since 2015, MED Institute has been focused on developing its partnership with medical device and biotech companies...
-

Uncertainty Analysis is an Important Part of Test Method Validation
“Testing” is a term that covers a vast range of activities. Not every test is a measurement;...
-

The Goals of Test Method Validation
Test method validation is a documented process that is used to confirm that the procedure to be...
-

Why do I need test method validation and what level of rigor is necessary?
The medical device industry has long understood the requirements related to process and software validation, however, US...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
