What do you do when regulatory requirements exceed reasonable engineering controls? – Part 2
In part one of this series, we talked about why regulatory requirements for medical device testing might exceed reasonable engineering controls. In this part, we’ll share the first of five real-life case studies in which MED Institute faced these kinds of discrepancies. In our first case study, there was a discrepancy regarding the required bifurcation tracking model anatomy for vascular stent testing. The company’s requirement was that the device must be advanced, deployed, and withdrawn in clinically relevant anatomy. The regulatory requirement was that the model should include “worst case” tortuosity and bifurcation radius. 
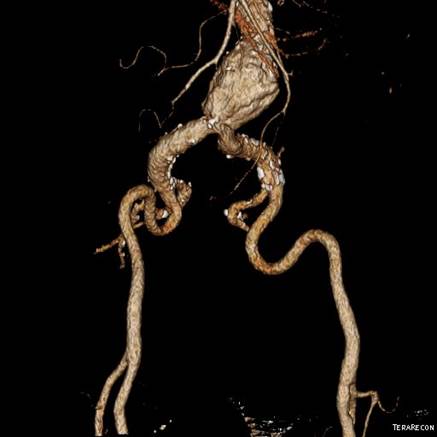
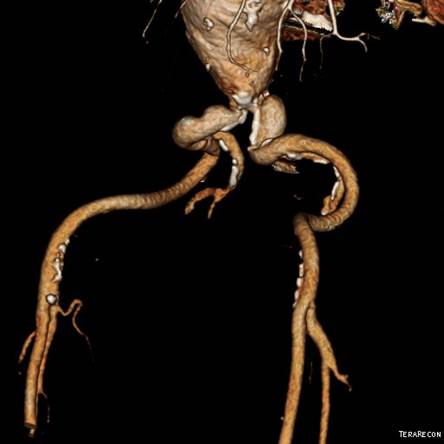 This difference between these requirements presented a few options. The first option is to use an anatomical model that the regulatory body agrees to as representing the worst case. This might seem like the easiest option, but it could suggest to the regulators that we don’t know the science behind our device. And in this case study, it also would require us to determine what the worst-case model is, which wouldn’t be an easy task. How bad is bad enough? How bad is the worst? The second option was to use literature and physician feedback to demonstrate to the regulatory body that the company’s model is clinically relevant and adequate. At MED Institute, the use of clinically relevant requirements is our preferred choice. The third option was to work with regulators to agree on a new solution. This option requires negotiation and might overlap with the second option. In this case, MED Institute collaborated with physicians and used clinical experience to develop an anatomical model, and negotiated with regulatory agencies to reach agreement regarding its clinical relevance. With the rapid pace of change in medicine, education is an ongoing process for both medical device companies and regulatory bodies. The conversations and negotiations that occur around testing requirements are part of the learning process.
This difference between these requirements presented a few options. The first option is to use an anatomical model that the regulatory body agrees to as representing the worst case. This might seem like the easiest option, but it could suggest to the regulators that we don’t know the science behind our device. And in this case study, it also would require us to determine what the worst-case model is, which wouldn’t be an easy task. How bad is bad enough? How bad is the worst? The second option was to use literature and physician feedback to demonstrate to the regulatory body that the company’s model is clinically relevant and adequate. At MED Institute, the use of clinically relevant requirements is our preferred choice. The third option was to work with regulators to agree on a new solution. This option requires negotiation and might overlap with the second option. In this case, MED Institute collaborated with physicians and used clinical experience to develop an anatomical model, and negotiated with regulatory agencies to reach agreement regarding its clinical relevance. With the rapid pace of change in medicine, education is an ongoing process for both medical device companies and regulatory bodies. The conversations and negotiations that occur around testing requirements are part of the learning process.
Get email about news, services, and events from MED Institute.
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
