-
Expanding MR Conditional labeling options using new laboratory equipment
We recently added new equipment to our MRI safety evaluations laboratory that can be used to provide...
-

On the Record: Compliance with 21 CFR Part 11 in Clinical Trials
What is 21 CFR Part 11? The United States Code of Federal Regulations (CFR) includes regulations for...
-

Radiopacity evaluation of a medical device
During the summer of 2022, we expanded into a new space to better serve our customers. This...
-

Scientific writers and clinical evaluation reports (CERs)
Scientific writers can help you with your clinical evaluation needs. Clinical evaluation is an ongoing requirement for...
-
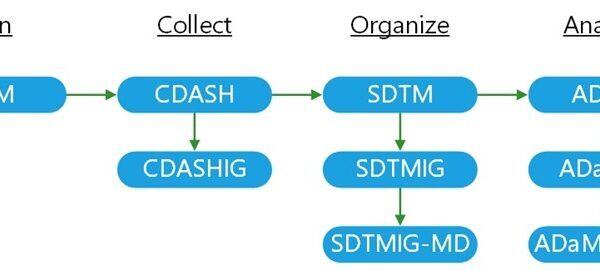
An Introduction to Implementing CDISC Study Data Standards
Importance of Study Data Standards Exchanging data globally has become much simpler as information technologies develop. However,...
-

ISO 10993-17 Revision
The new version of ISO 10993-17 is expected to be published very soon. This is a major...
-

Effective management of your clinical trial budget
Successfully executing a clinical trial is a large undertaking that requires strategic planning and cooperation. Even if...
-
AIMD MRI safety
We recently updated our website to highlight our capability of evaluating the MRI safety of active implantable...
-

Mid-Sized CRO Benefits
If you have decided to seek a CRO for a forthcoming study need, there are many benefits...
-

Is your CRO providing the customer service and flexibility you need?
Over the last several years, there have been rapid changes in the industry. Most notably, there has...
-

RF-Induced Heating of AIMDs
Next month, we will be presenting at the National Institutes of Health (NIH) as part of the...
-
What is a contract research organization (CRO)?
A Contract Research Organization (CRO) is a company that provides clinical trial services for the pharmaceutical, biotechnology,...
OUR COMMITMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business.
For client surveys sent since 2024, we received ratings of 4.99/5 (16).
